PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
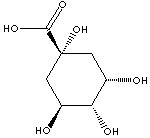
C1[C@@](C[C@H]([C@@H]([C@@H]1O)O)O)(O)C(=O)O
CLASSIFICATION
Cyclic polyol
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - Quinic acid
Wikipedia Linking - Quinic acid
Google Scholar Search - Quinic acid
U.S. National Library of Medicine - Quinic acid
PubChem Compound Summary - Quinic acid
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Quinic acid
http://www.ebi.ac.uk/chebi/ - Quinic acid
http://www.ncbi.nlm.nih.gov/ - Quinic acid
Material Safety Data Sheet - Quinic acid
EPA - Substance Registry Services - Quinic acid
Local:
Quinic acid is a white crystalline compound of nonnitrogenous acid found in various plants, such as cinchona bark, coffee beans, leaves of tobacco and carrot, and etc. It is soluble in water, alcohol and glacial acetic acid but insoluble in ether; melting at 162 C. It has two fuctional groups in the same molecule, hydroxyl groups and a carboxylic acid group which are optically active. They can yield various kinds of esters and salts. It belongs to the class of cyclitol, a polyhydroxylated cycloalkane containing at least three hydroxy groups in the ring at different positions. Examples of cyclitol are inositol and shikimic acid. The most important feature of cyclitol is that there are chiral isomers, key intermediates in the biosynthesis of aromatic compounds in living metabolism. Quinic acid is widely used as a chiral building block for the synthesis of pharmaceuticals. An example of end product is an antiviral drug oseltamivir, a neuraminidase inhibitor used in the treatment and prophylaxis of both influenza A and influenza B.
APPEARANCE
ASSAY
98.0% min
OPTICAL ROTATION
Hazard Symbols: , Risk Phrases: , Safety Phrases: 24/25